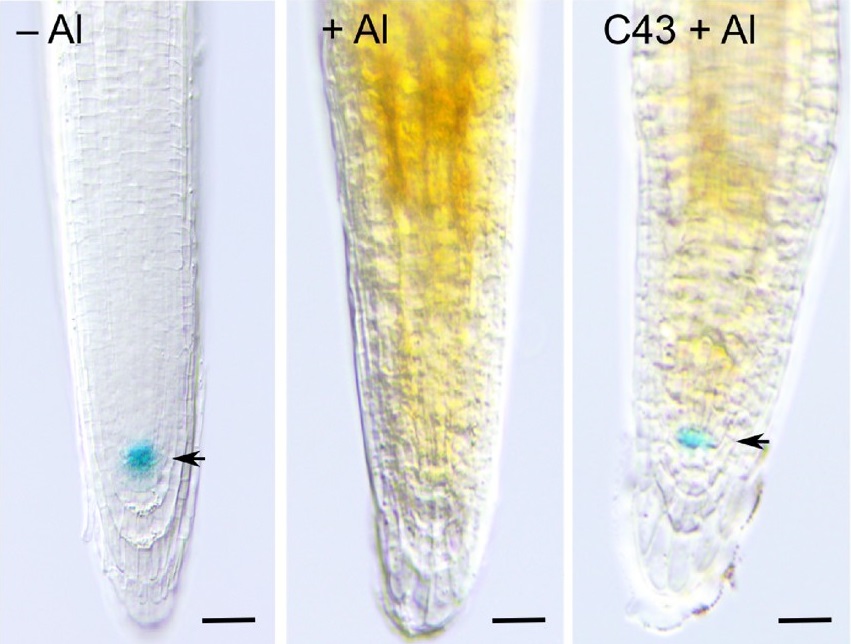
Genome integrity of cells is threatened by DNA damage caused by environmental and endogenous stresses. To cope with these stress conditions, cells have developed a set of surveillance mechanisms that monitor the status and structure of DNA during cell cycle progression. In fission yeast and mammals, DNA damage activates the signalling kinases ataxia telangiectasia mutated (ATM) and Rad3-related (ATR) that simultaneously turn on DNA repair complexes and arrest cell division, allowing cells to repair damaged DNA before proceeding into mitosis.
Because of their sessile lifestyle plants are more frequently exposed to adverse environmental conditions like drought, heat and toxic compounds in the soil, all of which can induce DNA damage through various mechanisms. Although ATM/ATR proteins appear to be conserved in plants, the downstream elements appear to differ significantly from mammals and yeasts. Through the use of both chemical and genetical screens we aim for the identification of plant-specific molecular components of the DNA damage signalling pathways, and subsequently study them to make plants more resistant to inhibitory growth conditions. In parallel, important signalling components are tested in maize. Using both in vitro and greenhouse experiments, these lines are tested for differential growth responses towards DNA damage-inducing environmental stresses and metals. Complementary, field trail experiments enable to test the impact of a natural environment on the growth of plants with impaired DNA damage sensing. Overall, we aim at a comprehensive understanding of the importance of DNA checkpoint control in maize under a natural environment at the morphogenetic, physiological, and genetic level, allowing to evaluate the possible use of checkpoint mutants to generate stress-resistant crops.